Figure 1
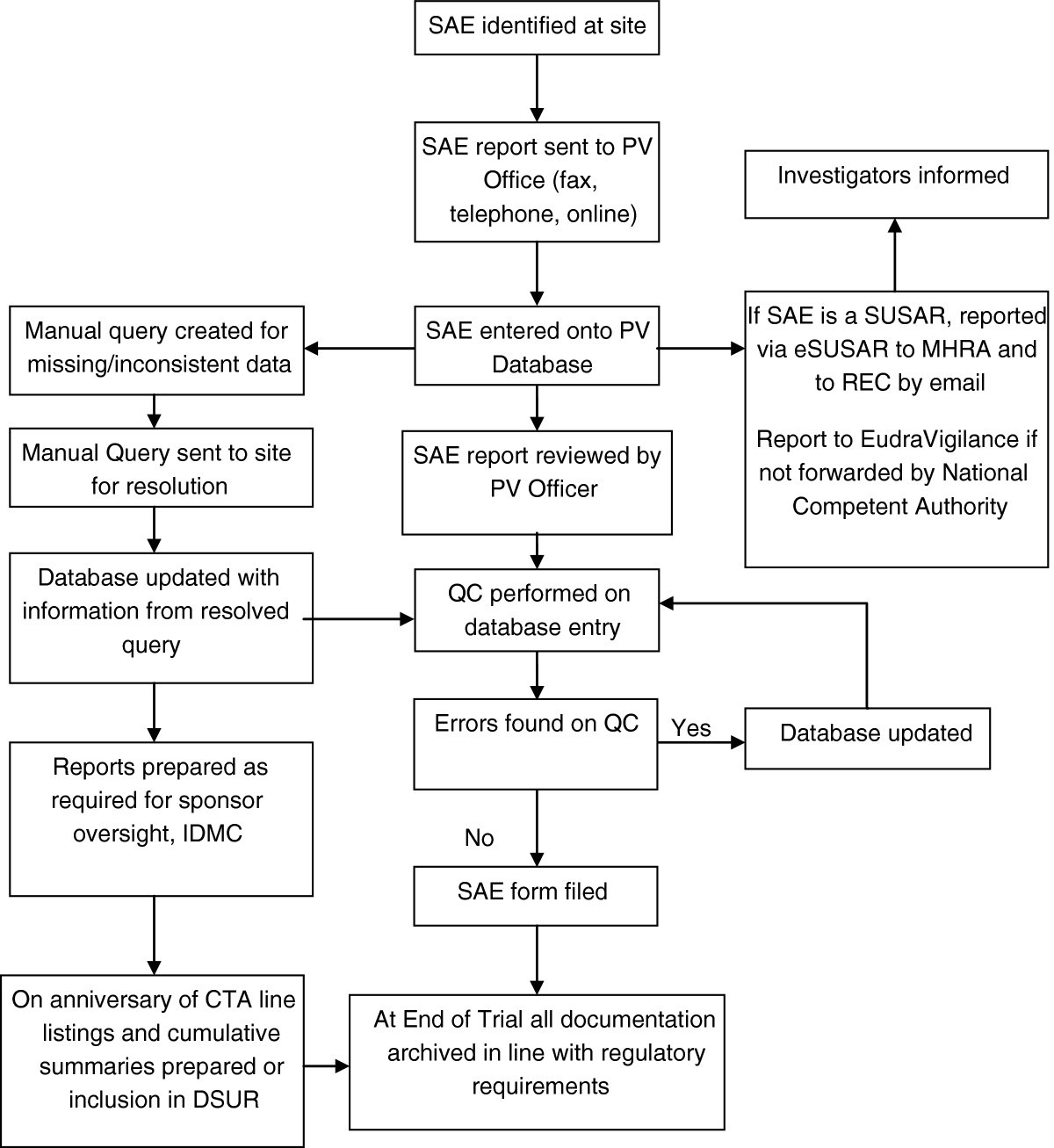
Flowchart of SAE data. CTA, clinical trial authorisation; DSUR, Development safety update report; IDMC, Independent data monitoring committee; MHRA, Medicines and healthcare products regulatory agency; PV, pharmacovigilance; QC, quality control; REC, Research ethics committee; SAE, Serious adverse event; SAR, Serious adverse reaction; SUSAR, suspected unexpected serious adverse reaction.
